-
97% Clinical response
more than 2x faster than tissue
Moving beyond tissue plasma concordance
Memorial Sloan Kettering Cancer Center, the Northern Cancer Institute of Sydney, and Resolution demonstrate clinical validation
While tumor tissue vs cell-free DNA in plasma concordance continues to dominate the liquid biopsy headlines, it really doesn't matter. What matters is that if you detect a mutation in plasma, does the patient respond to a plasma-directed therapy. That was the simple idea behind this study.
Read more
Clinical Validation
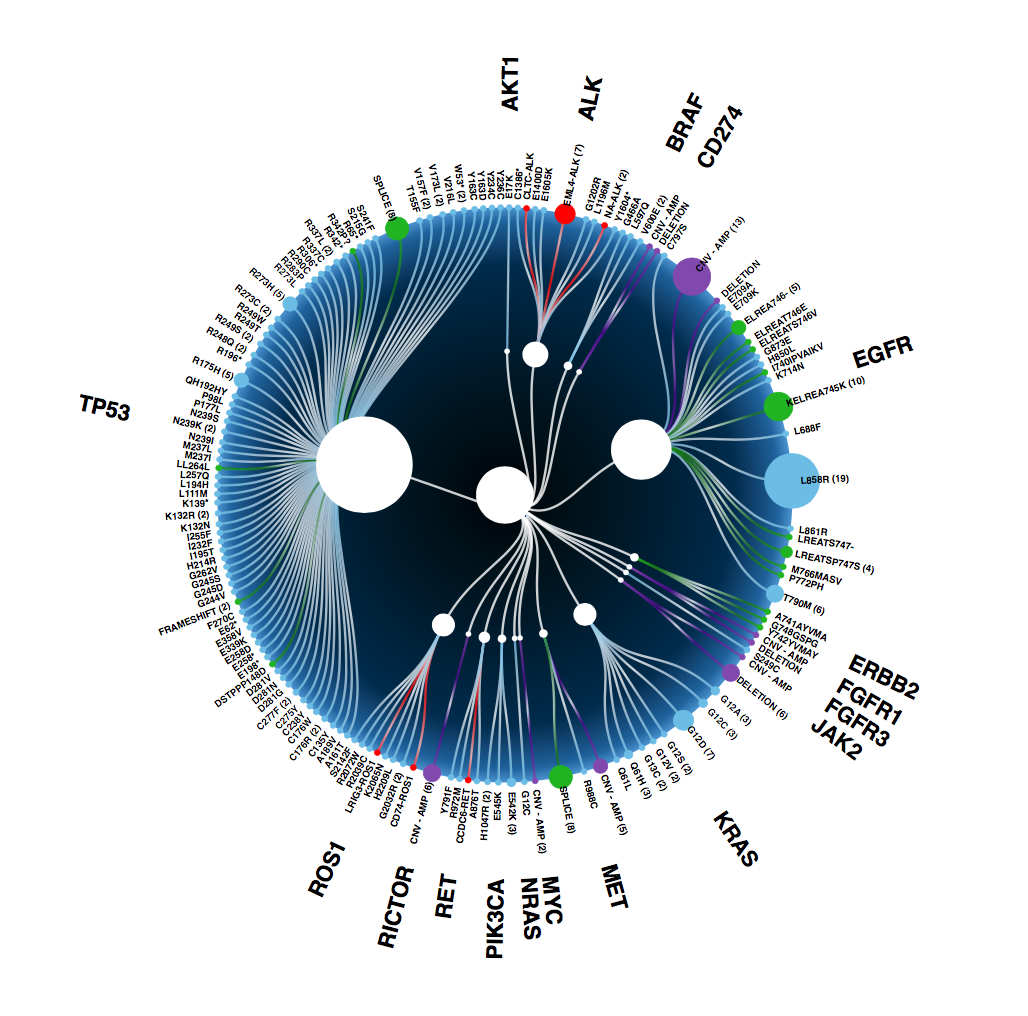
34 of the 35 evaluable patients who had plasma-directed therapy selection showed clinical response. That's 97% of patients responded. At the end of the day, that is all that matters. Tissue vs plasma concordance is irrelevant when you have clinical response based on plasma. We want to move the liquid biopsy discussion out of concordance and into the light of clinical response.
Patients who responded had EGFR exon19, EGFR T790M, EGFR L858R, EML4-ALK fusions, MET exon 14 skipping, BRAF V600E, RET fusions, ERBB2 insertions amongst others.
Time Matters
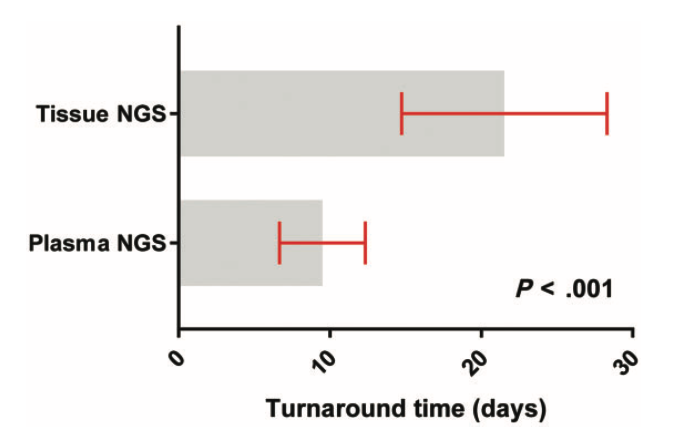
The Resolution ctDx-Lung test returned results more than twice as fast as the MSK-IMPACT tissue NGS assay. Results were successfully completed in 210 patients with a median turn-around-time (TAT) of 9 days from the time of blood draw to the time the primary investigator received the report.
MSKCC discusses the impact on their patients
"We’re very pleased that our study showed the value of a liquid biopsy. We hope that such tests will eventually become an integral part of care to help many more patients," — Dr. Bob Li
MSKCC wrote further about our study in this post: "Liquid Biopsy Is Effective at Guiding Treatment of Lung Cancer, Study Finds" Read more